EMA say: day zero starts at receipt of physical/hard copy local journals! Implications: contradictions with GVP, low impact on patient safety, higher costs for MAH
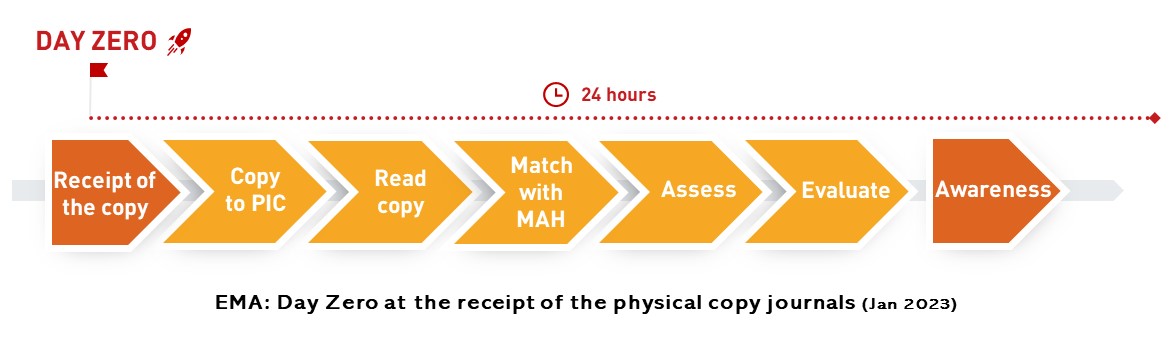
EMA say: day zero starts at receipt of physical/hard copy local journals! Implications: contradictions with GVP, low impact on patient safety, higher costs for MAH
Posted at 16:18h
in
Other
by ComFit Europe
EMA posted the following interpretation of the Pharmacovigilance Inspectors Working Group in January 2023:
“For ICSRs, medical literature containing the minimum criteria, the clock starts (day zero) on the day when
the physical/hard copy local journal is received by the organisation/Marketing authorisation holder/Applicant,
and this should be considered equal to performing a literature search in an electronic database.”
At present the general understanding is that day zero starts when the assessment of an article comes to a result. And the relevance and challenge of the topic is that day zero tends to be equal a 24-hour constraint in literature monitoring at most subsidiaries of MAHs:
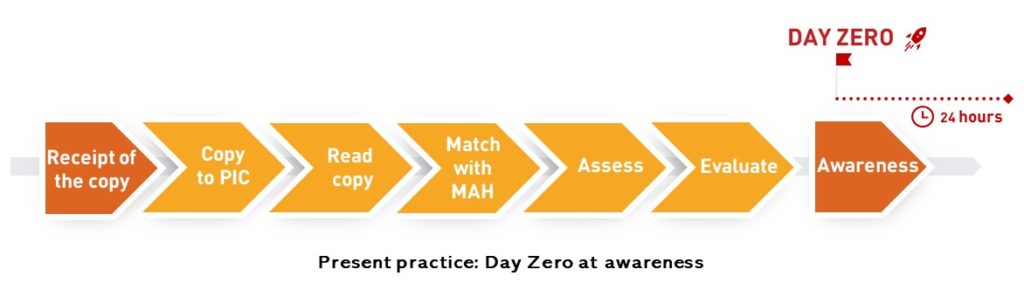
This is what EMA suggests:
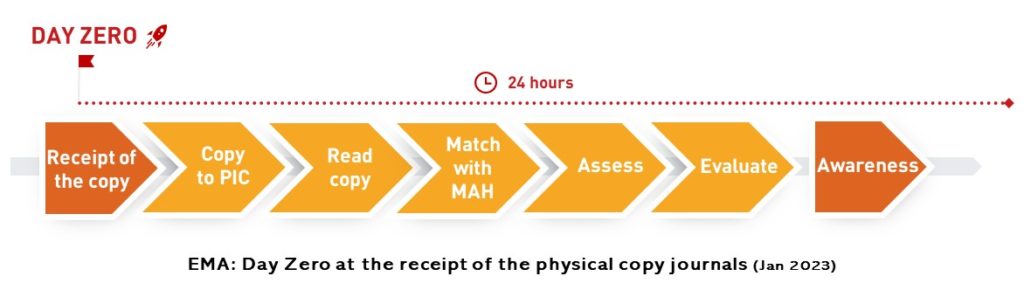
It is possible to live up to a 24-hour processing of hard copies starting with receipt of the copy, but due to the cut in time of the present practice and unpredictability of the exact receipt as well as the unpredictable number of articles in the copies, this interpretation implies to grow considerably the resources for monitoring. What’s the benefit from the point of view of patient safety and who will pay for that?
I. The interpretation of EMA does not seem to be in harmony with GVP. Here are the arguments:
A. Receipt time of the physical copies cannot be considered as the awareness of the minimum criteria due to the time span between the two points
Awareness supposes that all articles were read, they were matched with the search terms of the given MAH, assessment was carried out and evaluation was made about the presence of an ICSR (or relevant information for therapeutic area about the active substance). The process that leads to the awareness requires time. Receiving a journal can only be the starting point of the process that can result in awareness. There is a time span between the receipt of copies and the end to the assessment, therefore the time of their receipt cannot be the same as the awareness of the minimum criteria.
B. EMA equal search in physical copy local journals to search in an electronic database, which is not right
This approach disregards that running a search in an electronic database brings targeted result while at hard/physical copies you have to spend time reading through a journal to get to the level of data from where the electronic search starts. It is a big difference between the two ways that in electronic database search you only occupy with articles that have relevance to your requirements. In hard/physical copies, first you have to find the articles related to your portfolio. What is seconds in an electronic database, it may take days in physical/hard copies, just think about an abstract book with 600 abstracts.
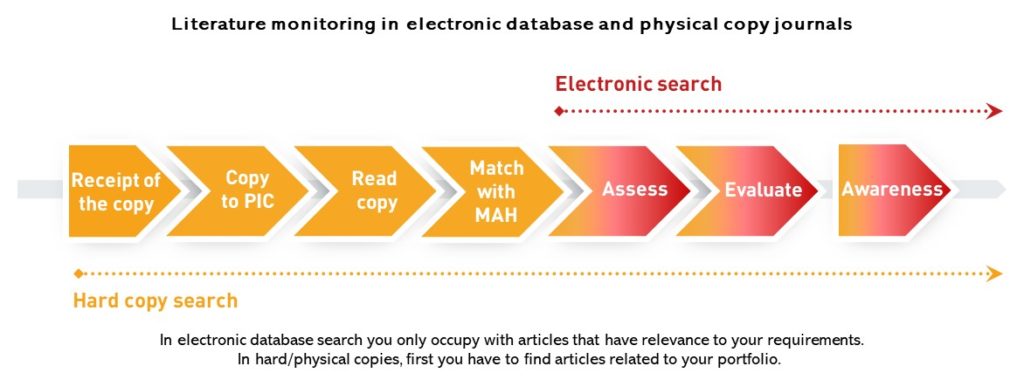
Another issue is what we mean by receipt of the journal. With hard/physical copies, the receipt of the copies at an organization usually means that the copy has arrived at the reception desk. It goes through administration before getting into the hands of the responsible person trained to assess articles. Please also consider the impact of a general tendency: what if the responsible person works in home office. All in all, at hard copies, there is a time lap compared to search in electronic database. Therefore, search in physical copies cannot be taken to be equal to monitoring in an electronic database from the point of view of time.
II. Low impact on patient safety and higher costs for MAH
It is hard, but possible to build a literature monitoring process that starts with the receipt of physical copies and meet 24-hour timeline in journals’ processing. The question is rather whether the risk-benefit assessment is favorable to aim at such a target.
C. The unpredictable arrival date of hard copy journals would mean big stand-by resources to keep
Hard copies can arrive at the organisations with postal service. Upon our 15-year-old experience in literature monitoring, the packaging and delivery of quarterly and monthly print copies mean +/-3 days compared to the date set by the publishing house. If you cannot know which day your organization will receive the medical journal exactly and you have a 24 hour processing constraint, you have to keep stand-by resources for the days when it may come. Having a group of trained, professionals on stand-by costs a lot, especially if you think of abstract books.
D. Abstract books – how to manage reasonably their ad hoc publishing and immense number of abstracts in 24 hours?
Generally speaking, a medical journal contains about 10 articles, they come more or less regularly and the article number is predictable by journal. You can calculate well how much resource you need for processing them. Abstract books can have 400 abstracts in one issue, they are rare to come and rather unpredictable in their arrival and their number of abstracts. 10 articles opposite to 400 abstracts in 24 hours. It is not the same resource. If you have to meet 24 hours with abstract books, too, you should keep significantly larger resources on stand-by all through the year.
E. Three-month old ICSRs in literature at least. Is a 24-hour deadline favourable from risk-benefit assessment?
A case in local literature or reference databases is at least three-month old when we are going to read about it in an article. 80% of medical journals are quarterly journals – 3 months to get to the reader. The 24-hour processing deadline does not seem to be reasonable while the original article drafts with the cases are waiting for months before publishing. And the delay starts with the doctors who are obliged to report cases, but they do not do it…..
III. Further comments:
F. How easier compliance with rules would be if EMA or GVP set at least the basics
– No less frequently than once a week” (VI.B.1.1.2. Literature reports)– why not saying simply once a week? What is the added value of no less frequently?
– “Scientific and medical publications” (VI.B.1.1.2. Literature reports) What is the definition of the terms – scientific and medical? How to interpret the conjunction and? Exclusion or inclusion? Why not medicine agencies take the role to publish the list of journals for monitoring? The medicine agency of Croatia and Hungary managed it.
G. GVP does not make a distinction of requirements for hard or electronic copies
Neither in this VI.B.7. Submission of individual case safety reports (ICSRs) nor in VI.App.2.7. Day zero, are there any references to differences as for physical/hard or electronic copies. These sections that EMA referred to as background of the interpretation speak about how to understand the terms day zero and ICSRs, no matter what the delivery format of the journal.
H.Reference databases contain local journals!
All journals have a publishing house, publishing houses have a headquarter which has an address in a country. In the end, all journals related to a country and can be considered local journals. Reference databases contain local journals. EMA’s interpretation speaks about the physical/hard copy local journal. However, the stricter deadline suggested in the interpretation can be the challenge of reference databases, too if they monitor journals that only deliver in physical/hard copies.





Sorry, the comment form is closed at this time.