Headquarters’ favourite

1.
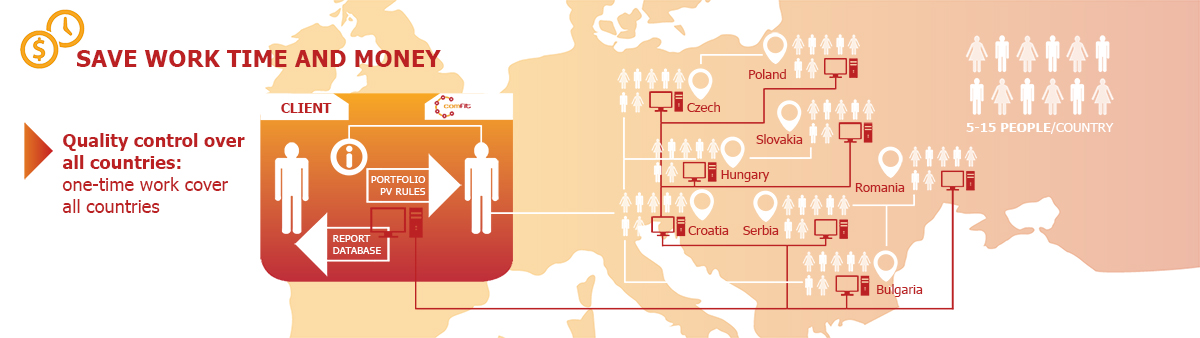
Quality control over countries. Our clients specify their PV requirements which we apply across countries. Thus, they get literature data in standardised format and quality.
2.
Every country’s patient related articles and other safety data from literature can be searched in the same database, which means simpler data queries, better and easier comparability for headquarters.
3.
Our multinational clients can check our working processes in all of the countries with one audit. They can spare work, time and money by not having to audit literature monitoring in the countries, one by one.
4.
One database, but individual reporting: we merge all data in one single database, still users can freely specify their report settings according to their individual needs. This means that affiliates can focus on their own country data, while headquarters or regional offices can have access to all.
5.
We are always ready to develop and introduce new processes so to live up to client’s special needs and expectations and to support them to accomplish their job according to their internal or external rules.