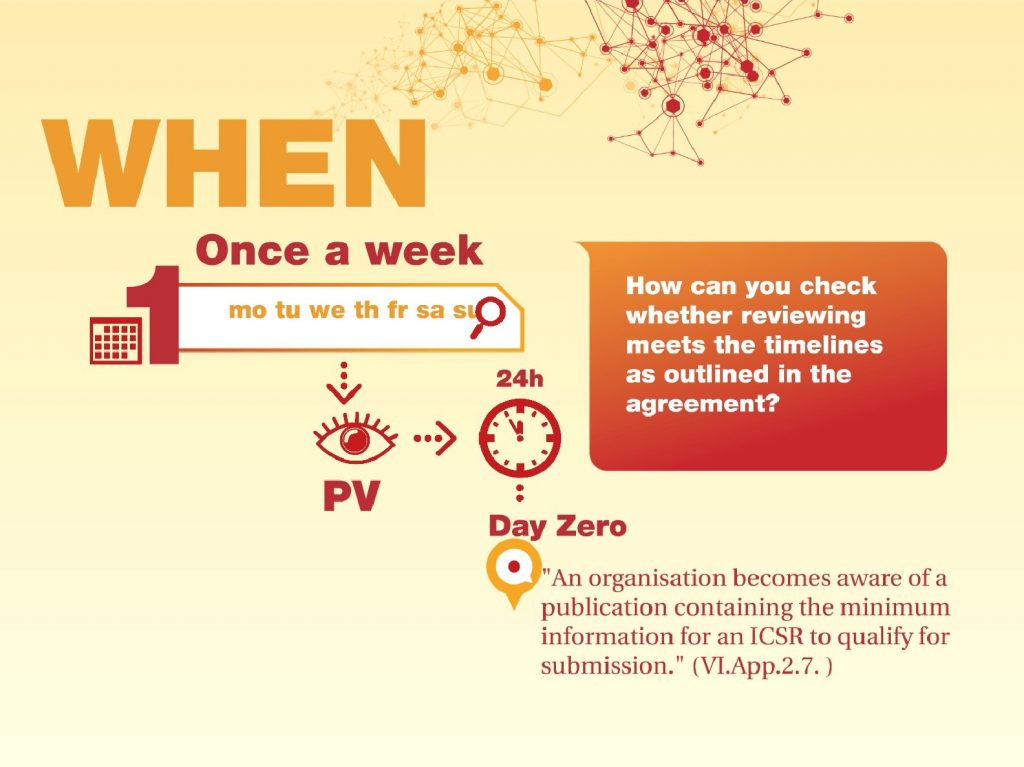
WHEN to carry out literature monitoring
One of the most challenging parts of reviewing is proving that the frequency and method of checking whether the literature monitoring activities are meeting the GVP timelines is efficient enough. Not only should you prove that you have covered ALL publications within a certain period of time without a miss, no matter if they have been published or delivered with delay, earlier or several simultaneously, but be able to provide proper explanation for any inconsistencies in publishing and when and how you have tracked them. If you want to find out more details on those challenges and how we answer to them in our ComFit software, please refer to the schemes we have prepared below:
This post is the sixth in a series. We believe that answering basic questions on search in literature such as which drugs, when etc., can guide us in building an effective literature monitoring system in compliance with GVP. We are going to zoom into seven questions and share our experience how we have successfully answered them at ComFit. Stay tuned!



Sorry, the comment form is closed at this time.