Medical literature monitoring Tag
Posted at 17:32h
in
Audit
by ComFit Europe
At ComFit, we have been collecting the questions of audits and quality checks for years and turn them into sources of further developments as well as to make them a part of a check-list for the next milestone. We have decided to write a summary...
Posted at 17:30h
in
Audit
by Comfit Europe
At ComFit, we have been collecting the questions of audits and quality checks for years and turn them into sources of further developments as well as to make them a part of a check-list for the next milestone. We have decided to write a summary...
Posted at 18:24h
in
Audit
by Comfit Europe
As we reached the end of our series on questions and GVP references to build and effective literature system, we would like to provide an overview of the topics we touched upon in the last few weeks as a guide to the 7 steps to...
Posted at 17:30h
in
Audit
by Comfit Europe
As GVP is clear that there is "no acceptable loss" (VI.App.2.3.4.) regarding the collection of PV data, ensuring the most reliable search strategy to cover ALL possible challenges and being able to justify its methods is crucial for the functioning of a literature monitoring system....
Posted at 17:57h
in
Audit
by Comfit Europe
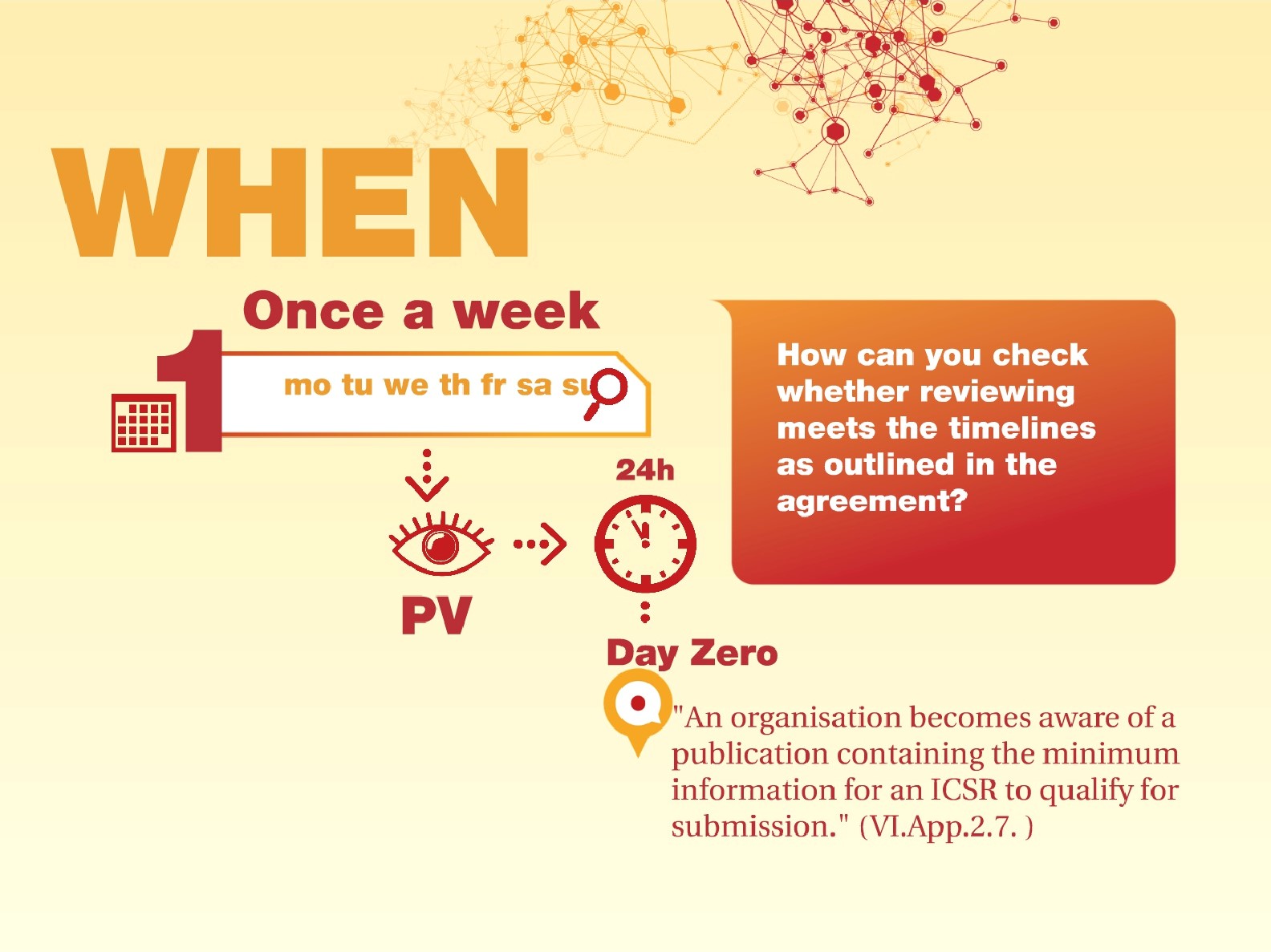
One of the most challenging parts of reviewing is proving that the frequency and method of checking whether the literature monitoring activities are meeting the GVP timelines is efficient enough. Not only should you prove that you have covered ALL publications within a certain period...
Posted at 17:30h
in
Audit
by Comfit Europe
The GVP dictates that detailed documentation and record keeping for extended period of time is crucial for proving that the literature monitoring process for collecting adverse drug events and special situations is being carried out in a systematic, well structured and reliable manner. A successful...
Posted at 16:55h
in
Other
by Comfit Europe
At the conference on 17th October 2018, organized by the Hungarian Regulatory Affairs Society (HURAS), the CEO of ComFit Europe Ltd., Katalin Timár-Horváth gave a presentation on the recommended list of local medical and scientific literature in Hungary. The recommendation list is under updating on...
Posted at 17:30h
in
Audit
by Comfit Europe
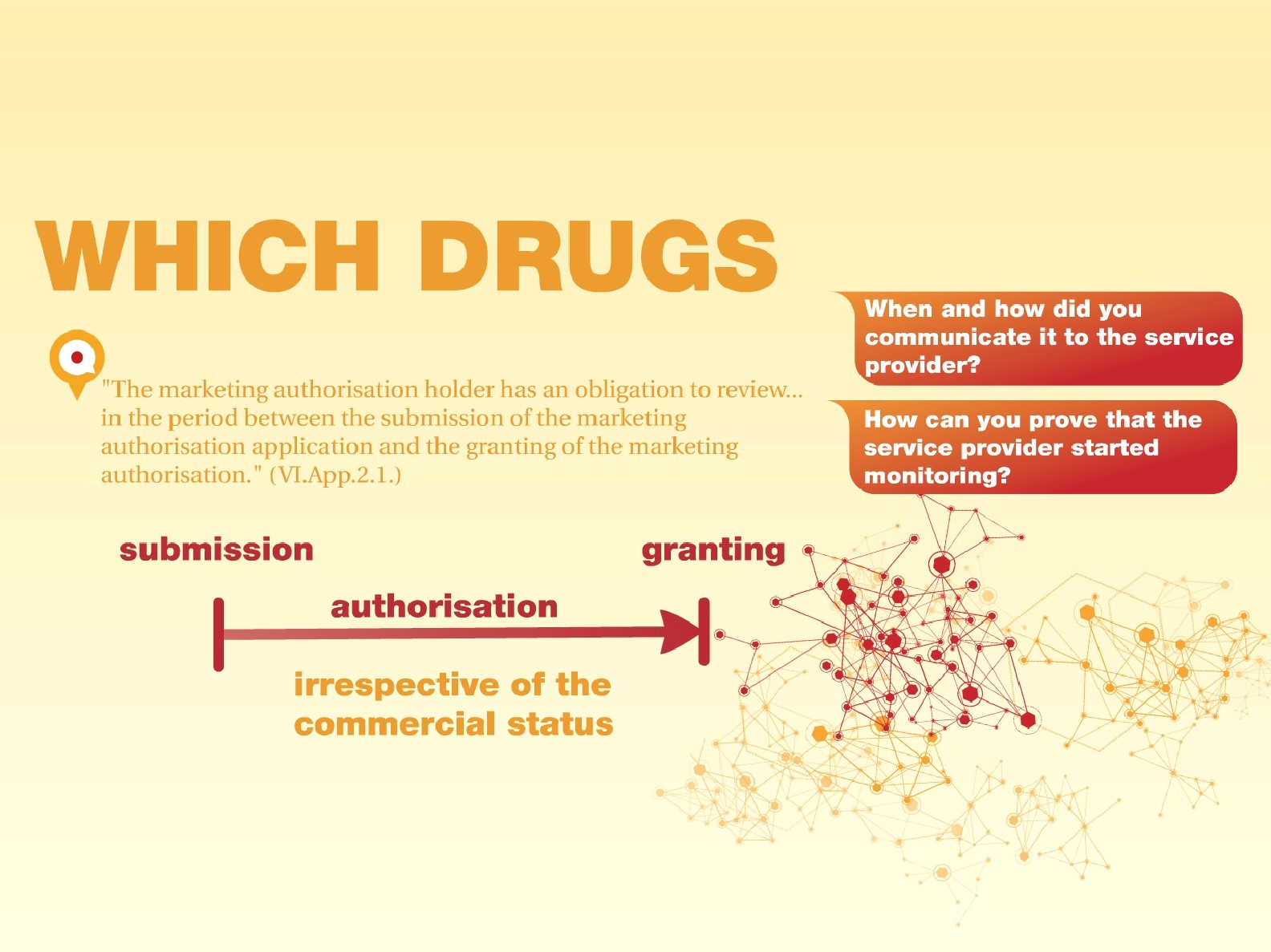
According to GVP "The marketing authorisation holder has an obligation to review...
Posted at 18:26h
in
Audit
by Comfit Europe
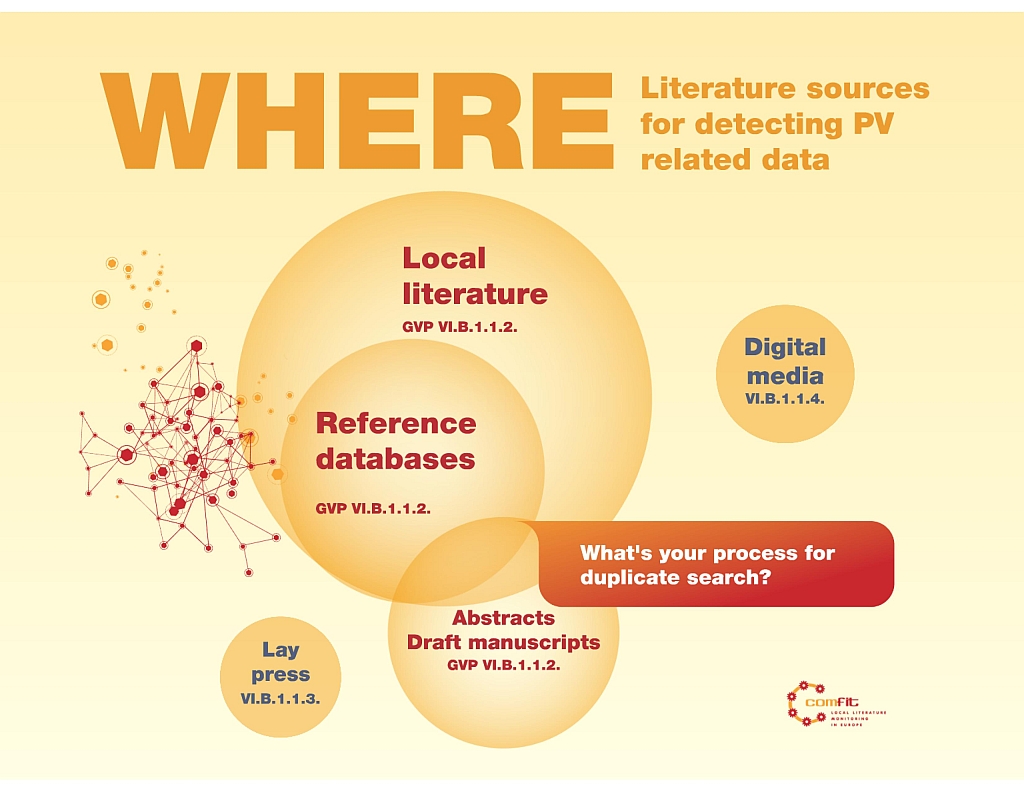
GVP says "The marketing authorisation holder should establish the most relevant source of published literature for each product." (VI. App 2.2. Where to look).
How do we define what a ‘relevant source’ is? It is an open question because authorities do not specify the sources to...
Posted at 16:51h
in
Audit
by Comfit Europe
As for literature, GVP says “It is best practice to have selected one or more databases.” (VI. App 2.2. Where to look) It mentions the need to monitor reference databases, local journals and to be attentive to find PV related data in lay and digital...